Researchers at the Southern Federal University (SFedU) have found a way to improve fuel cells, making them more affordable and durable. Their new catalyst based on platinum and copper accelerates the reactions needed to generate electricity and solves the problem of high material costs.

Fuel cells convert chemical energy into electricity, but their widespread use is limited by the high cost of platinum and the short lifespan of catalysts. The SFedU team studied how bimetallic nanoparticles based on platinum and copper behave in conditions close to real ones. The new material showed 5.5 times higher activity than commercial counterparts and retained 67% efficiency after 30,000 test cycles.
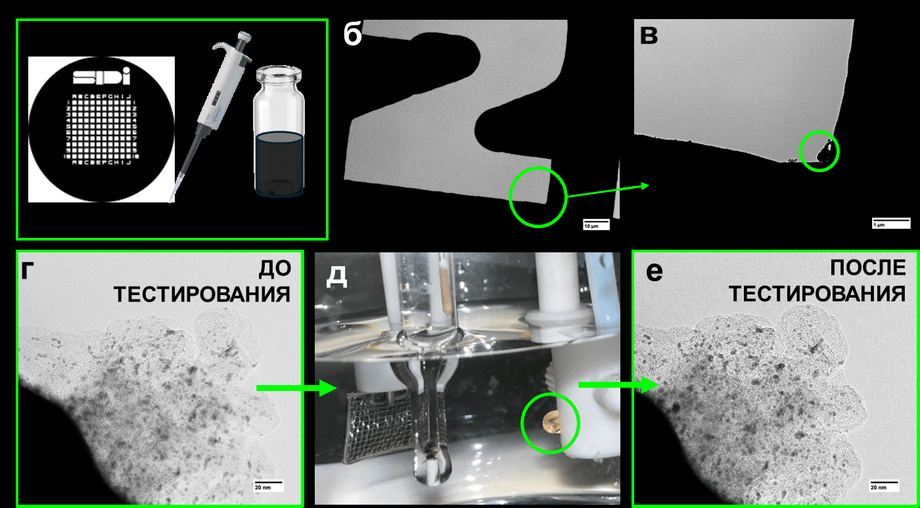
The key research tool was the IL-TEM method, implemented on a JEOL JEM-F200 microscope. Together with SEI (secondary electron imaging) technology, it allowed scientists to observe changes in nanoparticles on a carbon substrate with atomic precision before and after testing.
In this work, in addition to the IL-TEM method, the advanced technology of "secondary electron imaging" (SEI) was also used. This method allows obtaining a highly detailed picture of the surface and detecting even minimal morphological changes in the sample.
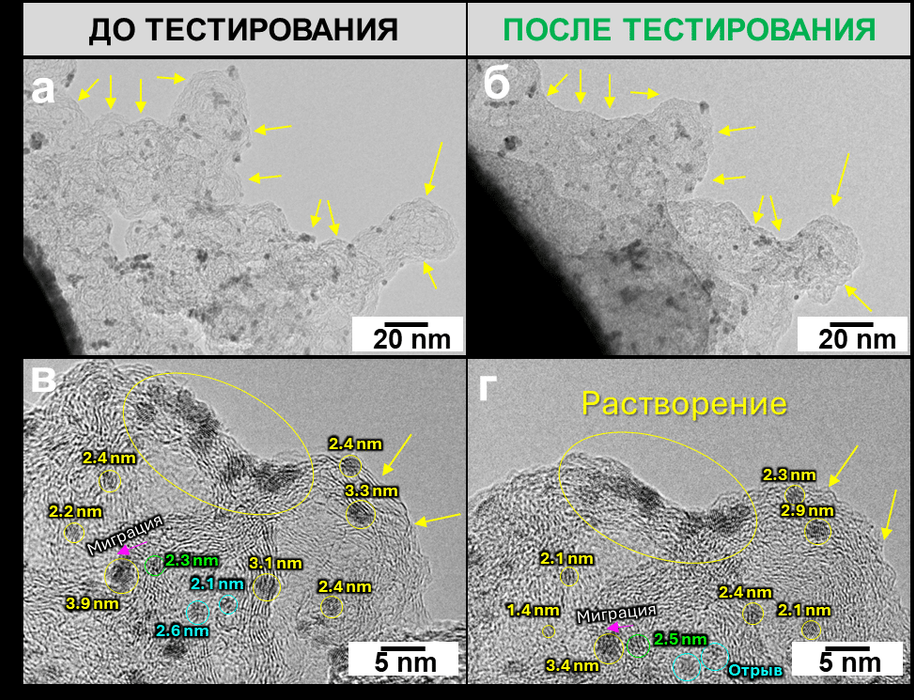
Three stress test methods showed different degradation mechanisms.
The material proved to be most stable in the standard catalytic protocol, corresponding to the stationary operation of the device, where the main degradation mechanism is the dissolution of nanoparticles without subsequent redeposition.
The study also revealed a problem: different groups of scientists use different testing conditions, which makes it difficult to compare results. Angelina Pavlets noted:
We conducted a collection and analysis of published studies on the application of the IL-TEM approach to assessing the degradation of complex catalytic systems and found that there is no systematization of data. Researchers use different testing conditions, which makes it difficult to compare the results obtained between different groups. Also, no more than two testing methods are used in the works for one sample, which, in our opinion, is not enough to certify the characteristics of catalytic systems.
The results are published in the journal Electrochimica Acta (Q1, impact factor 6.6). The work was supported by the Russian Science Foundation (project No. 24-79-10162).
We hope that the results of the microstructure transformation of a complex catalytic system presented by us will contribute to the development of this area and encourage scientists to comprehensively study objects, including using several testing methods in combination with the evaluation of the same local areas of the catalyst before and after testing.
The new catalyst reduces the platinum content by adding copper, making production cheaper. Compared to other catalysts, such as pure platinum ones, the SFedU development requires less precious metal and withstands loads better.
Read more materials on the topic:
TRINITI and MEPhI are creating a training center for thermonuclear energy











