A team of students from the Moscow Aviation Institute (MAI) is creating an innovative insulin pump fully controlled via a mobile application. The device is designed to mimic the functions of a healthy pancreas, providing continuous delivery of insulin in microdoses throughout the day. To date, there are no analogues with a similar degree of integration into Russian medical practice on the domestic market.
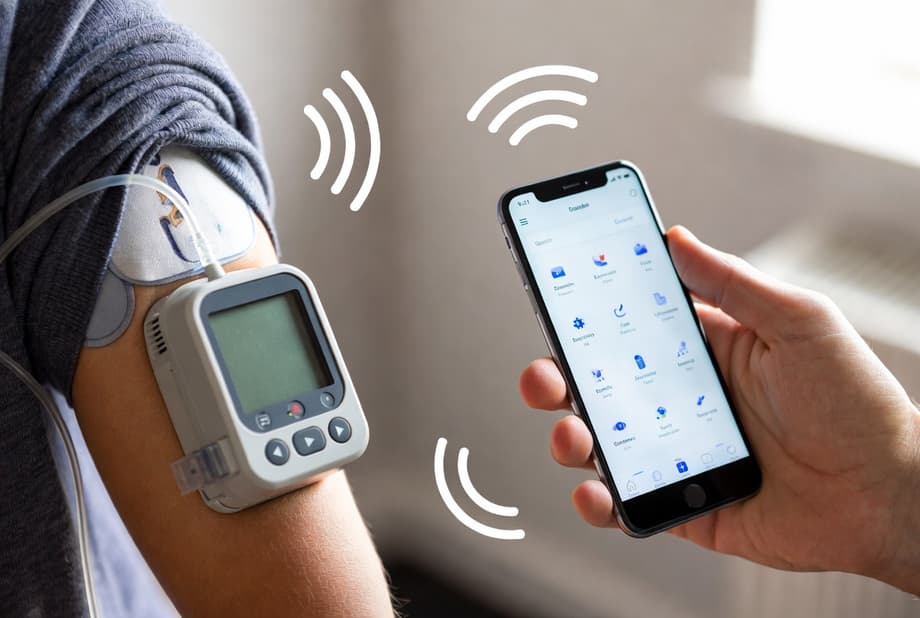
Our device will work wirelessly, which is very convenient for patients, and all control will be carried out via a smartphone. The mobile application analyzes glucose levels, helps calculate the insulin dose, provides personalized recommendations, and allows you to monitor treatment in a convenient format. You could say that we are creating not just a device, but a digital medical solution, maximally adapted to the needs of our doctors and patients.
According to Anisimov, the development aims not only to improve glucose control in patients with diabetes mellitus but also to reduce psychological stress: the absence of wires and the use of a familiar smartphone make the device inconspicuous in everyday life. This initiative is especially relevant against the backdrop of the need to ensure the availability of vital digital solutions in the context of possible restrictions on internet access and "white lists."
As a reminder, the issue of the lack of Russian software for diabetics has previously been raised on a direct line with Russian President Vladimir Putin.
The key advantage of the new pump is its deep adaptation to Russian clinical protocols. The system will include a recommendation module for insulin dosing, based on standards approved by the Ministry of Health of Russia. This fundamentally distinguishes the development from foreign analogues, such as the Chinese Medtrum TouchCare, and increases the safety of use in domestic conditions.
The project is being implemented with the support of one of the leading Russian manufacturers of glucometers, with whom the team is closely cooperating at the design stage. Full technical and legal preparation for clinical trials should be completed within a year.











